
Contents




|
Programme
Contents 


|
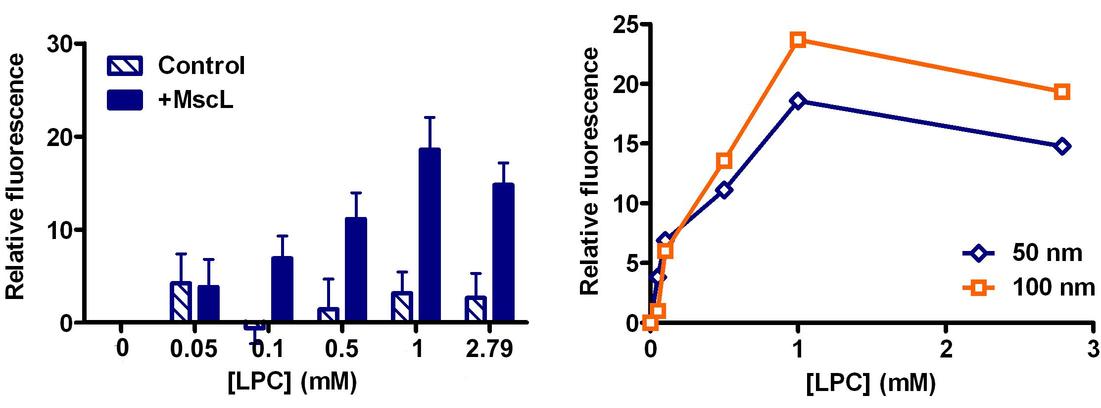
The bacterial mechanosensitive channel of large conductance (MscL) acts as an osmotically-activated nanovalve, allowing bacteria to respond to hypo-osmotic stress by opening nanometer-size channel pores. Significant insights into the underlying mechanism of channel activation and opening by membrane tension have been obtained for individual MscL channels reconstituted into artificial liposomes using patch clamp, electron paramagnetic resonance (EPR) and fluorescence spectroscopy in combination with computational modeling of channel dynamics during channel opening (Perozo et al., 2002; Corry et al., 2005; Betanzos et al., 2002). Given the relatively large size of the MscL pore (>25 Å), we have investigated its suitability for use as a nanovalve enabling controlled release of liposome-encapsulate particulates. Liposomes present one of the major forms of particulate drug carriers and provide an excellent method of encapsulation of highly toxic drugs, for example. In this study we have describe methods for generating small liposomes of uniform size based on a combination of liposome extrusion techniques and continuous sucrose gradient centrifugation. In addition, we demonstrate that MscL reconstituted into these liposomes may be used as a nanovalve for controlled release of small molecules including the self-quenching fluorescent dye 5,6-carboxyfluorescein (CF). CF release is regulated by the MscL-activating amphipath L-α-Lysophosphatidylcholine and exhibits a dependence on liposome size, amphipath concentration and protein-to-lipid ratio (see Figure).
Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B. (2002) Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature 418: 942-8.
Corry B, Rigby P, Liu ZW, Martinac B. (2005) Conformational changes involved in MscL channel gating measured using FRET spectroscopy. Biophysical Journal 89: L49-51
Betanzos M, Chiang CS, Guy HR, Sukharev S. (2002) A large iris-like expansion of a mechanosensitive channel protein induced by membrane tension. Nature Structural Biology 9:704-10.